R-TECH Materials received a water-bearing boiler tube which had been located in an area of high heat flux with the request to determine the cause of failure. The tube contained treated boiler feedwater at a temperature of 250ºC and a pressure of 60 bar.
Examination of the internal surface revealed the presence of a thick deposit in a localised area of the internal surface (Figure 1). This surface corresponded to the highest heat flux. When taking a section through this deposit, significant metal loss was evident directly beneath the deposit (Figure 2).

Figure 1 – thick deposit at internal surface in a localised area

Figure 2 – thick deposit at internal surface associated with metal loss
The deposit associated with the metal loss was porous, consisted of multiple layers (Figure 3) and exhibited copper particles throughout the layer (Figure 4). An analysis of the deposit showed it to consist of magnetite, a small amount of hematite, chlorine, sodium and copper. The microstructure was considered acceptable for the material and there was no evidence of any overheating.
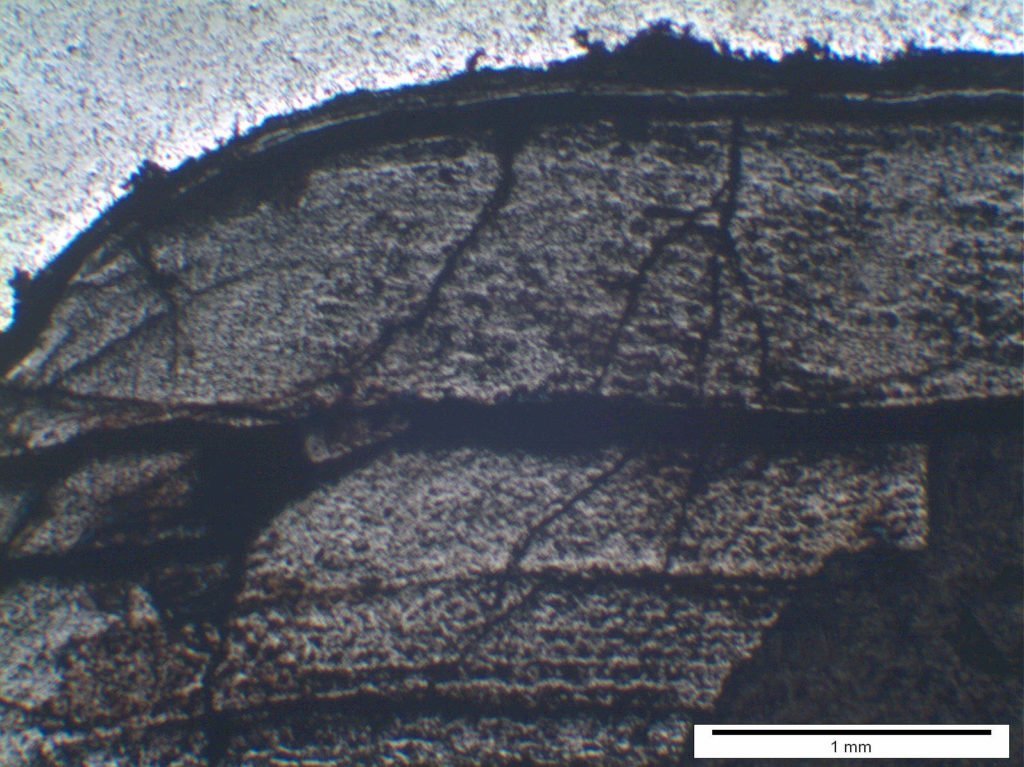
Figure 3 – multi-layered deposit at internal surface
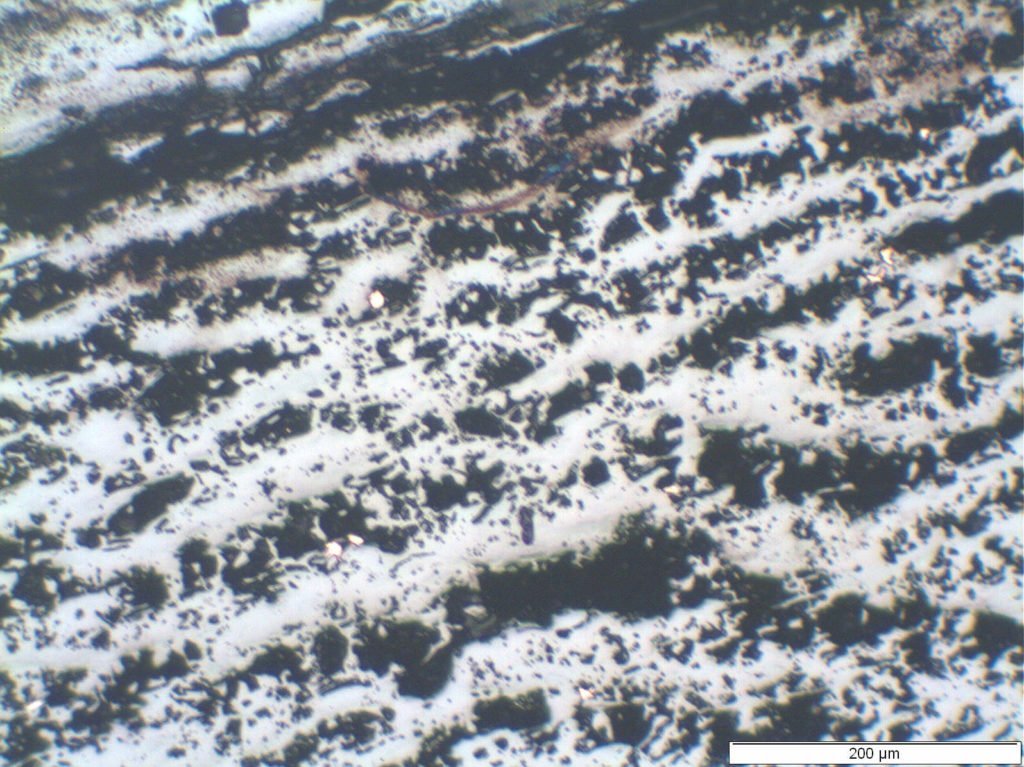
Figure 4 – copper particles within the deposit
It was deduced that the cause of failure was attributable to caustic gouging which occurs in the presence of a caustic species, typically sodium hydroxide. Caustic corrosion leads to irregular wall thinning or gouging of the tube until the resultant cross section cannot withstand the applied stress and rupture occurs.
A means of concentrating corrosive species is required for this type of corrosion. This can occur as a result of departure from nucleate boiling (DNB), which is caused by inadequate heat flow. This is where bubbles of steam nucleate at points on the metal surface. As these steam bubbles form, minute concentrations of boiler water solids will normally develop at the metal surface. The rate of bubble formation is said to exceed the rinsing rate and as a result caustic and other dissolved or suspended solids will concentrate. A similar situation also occurs when deposits shield the metal from the bulk water. Steam then forms underneath these thermally insulating deposits and escapes, leaving behind a corrosive residue that can deeply gouge the metal surface. Deposits can accumulate at any location of the tube.
An alternative mechanism is that the magnetite film loses its protective nature as a result of localised high heat input. This is then followed by the formation of a non-protective magnetite scale as a result of a reaction between the metal and oxygenated species. The corrosive species is then concentrated due to the presence of this porous magnetite layer.
A failure analysis of this type is a valuable and informative tool to reduce the likelihood of a repeat incident which improves safety and productivity, reduces the risk of unplanned outages and enables evolution towards a better product.